We are given that the density of ethanol is 0.789 g/mL. Density is defined as:
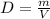
Where:
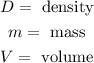
Since we are asked about the mass we will multiply the formula by "V" on both sides:
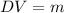
Substituting the values we get:
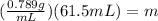
Solving the operations we get:
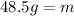
Therefore, the mass of ethanol is 48.5 grams.