ANSWER
The mass of the first product (calcium) in grams is 100.195 grams
Step-by-step explanation
Given that
The mass of calcium nitrate is 410.0 grams
Step 1; Write the balanced equation of the reaction
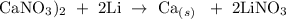
In the above reaction, 1 mole of calcium nitrate reacts with 2 moles of Li to produce 1 mole of Ca and 2 moles of LiNO3
Step 2; Find the mole of calcium nitrate using the below formula
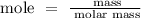
Recall, that the molar mass of Ca(NO3)2 is 164.1 g/mol
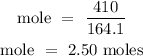
The moles of Calcium nitrate is 2.50 moles
Step 3; Find the mole of calcium using stoichiometry ratio
Since calcium is the first product of the reaction, hence, the number of moles of calcium can be calculated below
1 mole of Ca(NO3)2 produced 1 mole of ca
Let the number of moles of calcium be x
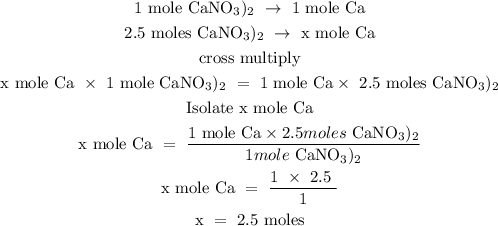
Therefore, the number of moles of calcium is 2.5 moles
Step 4; Find the mass of Ca using the below formula
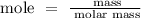
recall, that the molar mass of calcium is 40.078 g/mol
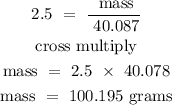
Therefore, the mass of the first product (calcium) in grams is 100.195 grams