Answer:
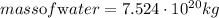
Explanations:
Given the following parameters;
Mass of CO2 = 4.6 * 10^20kg
Mass of CO2 = 4.6 * 10^23 grams
Determine the mole of CO2
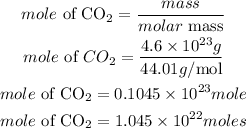
Since there are two atoms of oxygen in CO2, the total moles of oxygen will be expressed as:
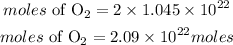
The reaction between Oxygen and Hydrogen is expressed as:
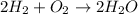
According to stochiometry, 1mole of oxygen produces 2 moles of water, hence the moles of water required will be given as;
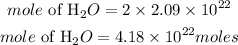
Determine the mass of water required:
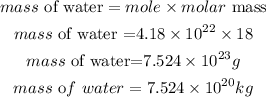
This gives the required total mass of water needed