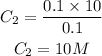Answer:
Trial 1; 0.102M
Trial 2: 0.094M
Trial 3: 10M
Explanations:
According to the dilution formula;
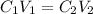
where:
• C1 and C2 are the initial and final concentration
,
• V1 and V2 are the initial and final volume
For the Trial 1:
• Concentration of HCl C1 = 0.1M
,
• Volume of HCl V1 = 10mL
,
• Volume of NaOH V2 = Vf - Vi = 10.3 - 0.5
,
• Volume of NaOH V2 = 9.8mL
Using the formula to determine molarity of base C2
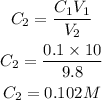
For the Trial 2:
• Concentration of HCl C1 = 0.1M
,
• Volume of HCl V1 = 10mL
,
• Volume of NaOH V2 = Vf - Vi = 20.9 - 10.3
,
• Volume of NaOH V2 = 10.6mL
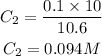
For the Trial 3:
• Concentration of HCl C1 = 0.1M
,
• Volume of HCl V1 = 10mL
,
• Volume of NaOH V2 = Vf - Vi = 30.0 - 20.9
,
• Volume of NaOH V2 = 0.1mL