First, let's see that we can extract the concentration of OH- because Ba(OH)2 is a base. Let's see the dissociation of this base:
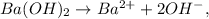
You can realize that we have 2 moles of OH-. The next step is to multiply this number of moles by the concentration (0.050 M):
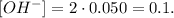
Remember that the formula of pOH is -log ( [OH-] ):
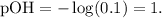
And with this result, we can find pH, using the formula:
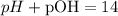
And we're going to obtain:
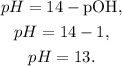
The pH of the solution would be 13, so the answer is (2).