We have in this case a precipitation reaction. Initially the reactants used are soluble in water so they will be in the aqueous state. The water does not intervene in the reaction, it is simply the medium where the reaction takes place. Now, when these two reactants react, two compounds will be formed, one soluble and the other insoluble, which will precipitate in the water.
The reaction that will take place will be:
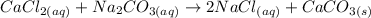
A compound will be formed that will precipitate in the water, this will be CaCO3 and a water-soluble salt, NaCl.