1) Mass percent
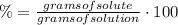
2) List known values
Sample: 0.965 kg
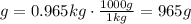
2) Plug in known values and solve for grams of solute.
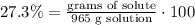
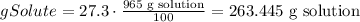
3) Convert grams of solute to moles
The molar mass of the unknown compound is 25.2 g/mol
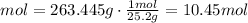
There are 10.45 moles of the unknown compound present in the sample.