Answer:
26.2L of O2 gas are required.
Step-by-step explanation:
1st) It is necessary to convert the 4.05L to moles. One mole of a gaseous substance occupies a volume of 22.4L, so wit a mathematical rule of three cwe can calculate the number of moles:
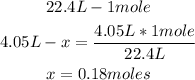
2nd) From the balanced reaction, we know that 2 moles of C4H10 gas react with 13 moles of O2 gas. So, with the 0.18 moles of C4H10 we need to calculate the amount of O2 gas that will react:
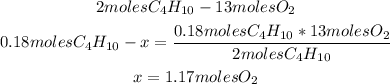
Now we know that 1.17 moles of O2 gas are required.
3rd) Finally, we have to convert the 1.17 moles to liters, using the 1mole:22.4L conversion:
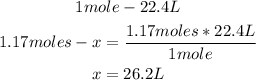
So, 26.2L of O2 gas are required.