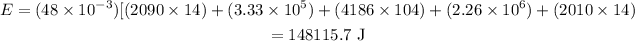Given data
*The given mass is m = 48 g = 48 × 10^-3 kg
*The given initial temperature of the ice is T = -14 °C
*The given temperature of the steam is t = 118 °C
*The specific heat of ice is c = 2090 J/kg °C
*The specific heat of water is s = 4186 J/kg °C
*The specific heat of the stream is 2010 J/kg ° C
*The heat of vaporization is 2.26 x 10^6 J/kg
*The heat of fusion is 3.33 x 10^5 J/kg
The formula for the energy is required to change a 48 g ice cube from ice at -14 °C to
steam at 118 °C is given as
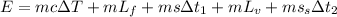
Substitute the values in the above expression as