Answer:
91.2 g of PCl3.
Step-by-step explanation:
What is given?
molecules of PCl3 = 4.00 x 10²³ molecules.
molar mass of PCl3 = 137.3 g/mol (you can calculate this using the periodic table).
Avogadro's number = 6.022 x 10²³ molecules/mol.
Step-by-step solution:
First, we have to convert from molecules to moles using Avogadro's number, which says that there are 6.022 x 10²³ molecules in 1 mol. The conversion will look like this:
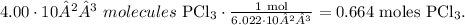
And the final step is to convert from 0.664 moles of PCl3 to grams using its molar mass which is given at the beginning (137.3 g/mol):
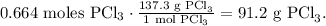
The answer is that we have 91.2 g of PCl3 in 4.00 x 10²³ molecules of PCl3.