INFORMATION:
Is given that
And we must find how much energy is consumed by thawing 4.3 g ice
STEP BY STEP EXPLANATION:
To find it, we need to multiply the mass of the ice by the Hfusion
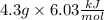
Since we need the energy, the answer must be in kJ. So, we need to use the molar mass to obtain the answer in kJ
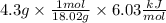
Finally, when we simplify the expression, the answer will be in kJ
ANSWER:
A. 4.3 g x 1 mol/18.02 g x 6.03 kJ/mol