As they don't give us data about the heat capacity of the soda, we will assume it as if it were liquid water.
To answer this question, we have to use the formula for calculation of heat.
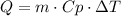
Where Q is the energy absorbed or released, m is the mass of the substance, Cp is the heat capacity and ΔT is the difference in temperature.
Replace for the given values to find Q:
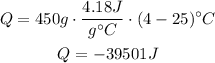
39501 joules are released when the soda is cooled.