ANSWER
The number of moles of the gas is 2.2 moles
Step-by-step explanation
Given thanks
The volume of the gas is 48.4L
To find the number of moles, follow the steps below
Recall, that 1 mole is equivalent to 22.4L/mol
Let x represents the number of moles of the gas
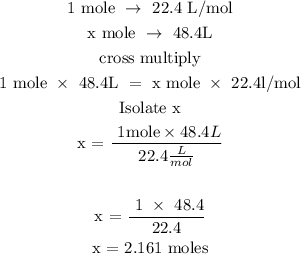
Hence, the number of moles of the gas is 2.2moles