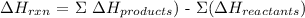Answer:
Step-by-step explanation:
Here, we want to give a definition
Delta H of a reaction is the change in ethalpy that occurs at the end of the reaction. It is the end result of heat changes that occur in a chemical reaction
It describes what occurs at the end of the reaction
For instance, it is negative for an exothermic reaction which indicates that heat is given off. It is positive for an endothermic reaction, meaning heat is absorbed
Mathematically: