Answer
Correct
Step-by-step explanation
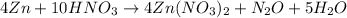
From the balanced chemical reaction, 4 moles of Zn reacted with 10 moles of HNO3 to produced 4 moles, 1 mole, and 5 moles of Zn(NO3)2, N2O, and H2O respectively.
Therefore, comparing the mole of Zn to HNO3, then the derived ratio is correct.