Given:
Initial volume:

Initial temperature:
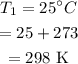
Final temperature:
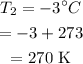
Mass of Helium gas:
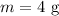
Therefore, the number of moles of Helium gas is given as,
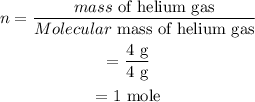
The ideal gas equation is given as,
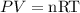
Here, P is the pressure and R is the universal gas constant.
Therefore,
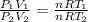
As, the pressure is same. Therefore,
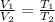
Therefore, the new volume is given as,
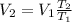
Substituting all known values,
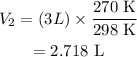
Therefore, the new volume of the gas is 2.718 L.