The question requires us to calculate the percent yield of HF in a reaction, given its actual yield (10.41g) and theoretical yield (12.79g).
The percent yield of a chemical process can be calculated as:
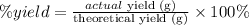
Thus, applying the values given by the question, we can calculate the percent yield of HF:
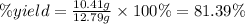
Therefore, the percent yield in the reaction mentioned is 81.39%. Note that the question provided values with four significant figures, thus our answer must also present four significant figures.