Answer:
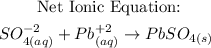
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction. In this step, we write the Molecular equation, including the phases of each compound:
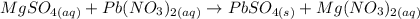
2nd) It is very usefull to write the Complete ionic equation, to identify the ionic form of each element in the reaction. In this step it is important to write only the ionic form of elements in aqueous phase:
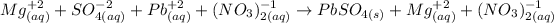
3rd) Finally, we can write the Net ionic equation, canceling the ionic species that repeat on both sides of the Complete ionic equation:
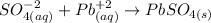
So, this is the Net ionic equation.