They give us the equation of the reaction already balanced:
CaO(s) + CO2 ----> CaCO3(s)
So we can proceed to do the calculations.
We will initially determine which is the limiting reactant and we will make the theoretical yield calculations based on this reactant. Finally we will find the percent yield by dividing the amount obtained by the chemist by the theoretical amount.
We will follow the following steps:
1. We calculate the moles of the reactants by dividing the given weight by the molar mass.
Molar mass CaO= 56.0774g/mol
Molar mass CO2= 44.01g/mol
2. We calculate the limiting reactant by determining which reactant has fewer moles. This is because the CaO to CO2 ratio is 1 to 1, meaning that the same moles of each reactant are needed to react.
3. We find the moles of CaCO3 by the stoichiometry of the reaction.
4. We find the grams of CaCO3 by multiplying the moles found by the molar mass, this will be the theoretical yield. Molar mass CaCO3=100.0869g/mol
5. We calculate the percent yield by dividing the amount found above by the current yield=19.4g CaCO3.
Let's proceed with the calculations.
1. Moles of reactants
Mol of CaO
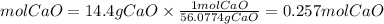
Mol of CO2
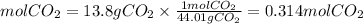
2. Limiting reactant
We have fewer moles of CaO, since the ratio is 1 to 1 this will be the limiting reactant, so the following calculations we will do with 0.257mol of CaO
Limiting reactant: CaO
3. Moles of CaCO3
The ratio CaCO3 to CaO is 1/1 so we have the same number of moles of CaO.
Moles of CaCO3 = 0.257mol
4. Grams of CaCO3 (Theoretical yield)
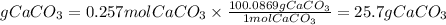
The theoretical yield of CaCO3 is 25.7g
5. Percent yield
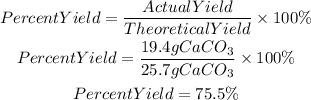
The percent yield of CaCO3 is 75.5%