Answer
0.03 moles
Step-by-step explanation:
Given:
The molar mass of the unknown = 161.49 g/mol
Mass of the unknown = 4,475.01 milligrams
Step 1: Convert the mass given from milligram to gram.
Note: 1 milligram = 1 x 10⁻³ gram
So 4,475.01 milligrams will be
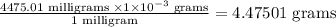
Step 2: Calculate the number of moles of the unknown.
The mole of the unknown element can be calculated by the given formula below:
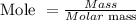
Substitute the values of mass and molar mass into the formula above:
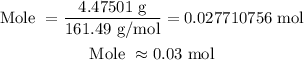
Therefore, there are 0.03 moles in 4,475.01 milligrams of an unknown element with a molar mass of 161.49 g/mol4