According to the Chemical equation 18 mols of water ar produced when 25 mols of Oxigen are consumed. Now we need to know how many mols of Oxigen are consumed in this example. The text give us the required information to solve this, but we need to use the ideal gas law:
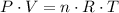
Where P is the pressure (1.30 atm), V is the volume (20L), T is the temperature in kelvin (263.15K) and n is the number of mols, what we want to calculate. Therefore we have to solve the ideal gas equation for n and use the provided data:
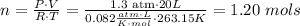
so 1.20 mols of O2 are consumed we have to use the conversion factor 18 mols of water/25 mols of O2 to obtain how many mols of water are produced:
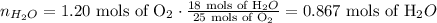
And the last step convert those mols into mass. To do that we need the molar mass of water, Mm=18 g/mol:
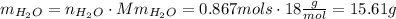
15.61 grams of water are produced