The first step to solve this problem is to write the chemical equation involved:
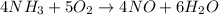
Use the mole ratio of ammonia to nitrogen monoxide which is 4:4 or 1:1 to find the moles produced by the given amount of ammonia:
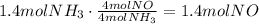
It means that the answer is 1.4 moles of nitrogen oxide.