ANSWER
The wavelength of the photon is 367.87 nm
Step-by-step explanation
Given information
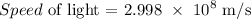
To find the wavelength of the photon absorbed, we will need to find the change in energy in transition using the below formula
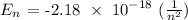
From the question, the energy states given are from n = 21 to n =2
So, we can calculate the value of energy below
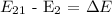
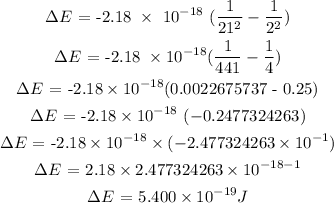
Since we have gotten the value of the energy, hence, we can now calculate the wavelength of the photon using the below formula

Recall,
h = 6.626 x 10^-34 J.s
c = 2.998 x 10^8 m/s
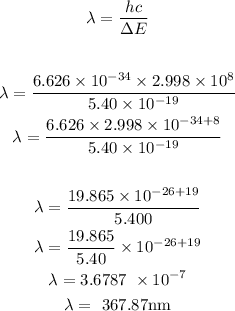
Hence, the wavelength of the photon is 367.87 nm