Step-by-step explanation:
Given;
We are given the general form of an exponential function and that is;
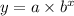
Required;
We are required to determine when it represents a growth and when it represents a decay.
Step-by-step solution/explanation;
The exponential function in its expanded form is given as follows;
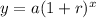
Take note of the following variables;
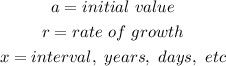
Hence, note also, that;
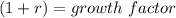
Note also that;
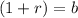
Therefore, if there is a growth, the formula would be;
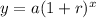
Which means;
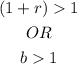
And if there is a decay, the formula would be;
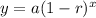
Which means;
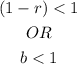
Therefore,
ANSWER:
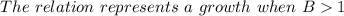
[tex]And\text{ }a\text{ }decay\text{ }when\text{ }0First is
option ASecond is option B