Answer
n=4
Procedure
Data
Sulfur has an atomic mass of 32.065 u
Fluorine has an atomic mass of 18.9984 u
To solve this problem we need to find the number of atoms of fluorine, this can be done using an equation as follows
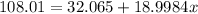
Solving for x we have
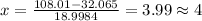
Hence as we can't have a decimal atom we consider 4 atoms of fluorine.