The correct statement is: "For a gas, pressure multiplied by volume is constant at constant temperature."
Boyle's Law describes the relationship between the pressure and volume of a gas at constant temperature. The correct statement is: "For a gas, pressure multiplied by volume is constant at constant temperature."
Boyle's Law is mathematically expressed as
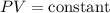 , where
, where
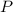 is the pressure, and
is the pressure, and
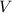 is the volume of the gas. This means that when the pressure of a gas increases, its volume decreases, and vice versa, as long as the temperature remains constant. In other words, the product of pressure and volume is constant for a given amount of gas at constant temperature.
is the volume of the gas. This means that when the pressure of a gas increases, its volume decreases, and vice versa, as long as the temperature remains constant. In other words, the product of pressure and volume is constant for a given amount of gas at constant temperature.
To understand this concept, consider a fixed amount of gas confined in a container. If the volume of the container is reduced (compression), the gas particles collide more frequently with the container walls, resulting in an increase in pressure. Conversely, if the volume is increased (expansion), the gas particles collide less frequently with the container walls, leading to a decrease in pressure.
Mathematically, Boyle's Law can be expressed as:
![\[ P_1V_1 = P_2V_2 \]](https://img.qammunity.org/2023/formulas/chemistry/college/1s4ghqxmdanulg11lou0cusmh8jnlq8sk1.png)
where
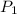 and
and
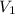 are the initial pressure and volume, and
are the initial pressure and volume, and
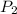 and
and
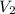 are the final pressure and volume.
are the final pressure and volume.
The correct statement emphasizes that the product of pressure and volume,
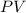 , remains constant when temperature is held constant. This is a fundamental principle that helps explain the behavior of gases under different conditions.
, remains constant when temperature is held constant. This is a fundamental principle that helps explain the behavior of gases under different conditions.