Answer:
Step-by-step explanation:
Here, we want to get the amount of heat transferred
We can get that with the following formula:
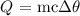
where:
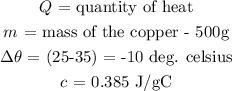
Substituting these values, we have it that:
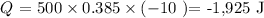
We have a negative answer as we are moving from a higher to a lower temperature which indicates a heat loss