Answer:
41.54 grams of oxygen are required to burn 13.5 g of acetylene
Step-by-step explanation:
The balanced reaction is:
2 C₂H₂ + 5 O₂ → 4 CO₂ + 2 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
- C₂H₂: 2 moles
- O₂: 5 moles
- CO₂: 4 moles
- H₂O: 2 moles
Being the molar mass of the compounds:
- C₂H₂: 26 g/mole
- O₂: 32 g/mole
- CO₂: 44 g/mole
- H₂O: 18 g/mole
By reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
- C₂H₂: 2 moles* 26 g/mole= 52 grams
- O₂: 5 moles* 32 g/mole= 160 grams
- CO₂: 4 moles* 44 g/mole= 176 grams
- H₂O: 2 moles* 18 g/mole= 36 grams
You can apply the following rule of three: if by stoichiometry 52 grams of acetylene react with 160 grams of oxygen, 13.5 grams of acetylene react with how much mass of oxygen?
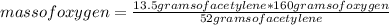
mass of oxygen= 41.54 grams
41.54 grams of oxygen are required to burn 13.5 g of acetylene