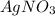Answer:
Step-by-step explanation:
When calculating an empirical formula from grams, divide each mass by their molar mass to convert your grams to moles.
63.5/107.7 = 0.5896 mol
8.2/14.01 = 0.5852mol
28.3/15.99 = 1.770 mol
Then you will divide all of your mol values by the SMALLEST number of moles. This gives you whole numbers that are the mole ratio (subscripts) of the empirical formula.
0.5896 mol/0.582 mol= 1
0.5852mole /0.582 mol= 1
1.770mol/0.582 mol= 3
So the empirical formula is