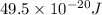Answer: The energy of a mole of photon associated with this frequency is
Step-by-step explanation:
The energy and frequency are related by :
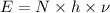
E = energy of photon
N = number of moles = 1
h = planks constant =
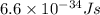
 = frequency =
= frequency =
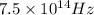
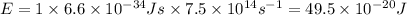
The energy of a mole of photon associated with this frequency is