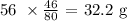Answer:
Step-by-step explanation:
The first thing we have to write here is the equation of reaction that forms rust
We have that as:
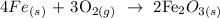
From the equation of reaction, 4 moles of iron react with 3 moles of oxygen to give 2 moles of rust
Now, let us get the actual number of moles
To get the number of moles of rust formed, we have to divide the mass of rust formed by the molar mass of rust
The molar mass of rust is 160 g/mol
The number of moles of rust formed is thus:
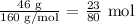
From the equation of reaction:
4 moles of iron give 2 moles of rust
x moles of iron will give 23/80 moles of rust
Mathematically:
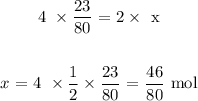
Now, to get the mass of the metal reacted, we have to multiply the atomic mass of iron by the number of moles above
The atomic mass of iron is 56 amu
Thus, we have the mass of the metal formed as: