Answer:
1.76moles
Explanations:
The number of moles of gas "n" is directly proportional to its volume "v". This can be expressed as:
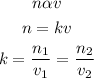
where:
n1 and n2 are initial and final moles respectively
v1 and v2 are the initial and final volumes
Given the following parameters
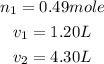
Required
Final number of moles "n2"
Substitute the given parameters into the formula
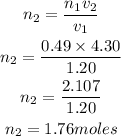
Hence the moles of gas added to increase the volume to 4.30L is 1.76moles