Let's find the number of moles of 4.14 g of H2SO4 using its molar mass:
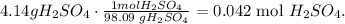
With this value, we can calculate how many moles of NaOH (base) we have to calculate its molarity. In the chemical reaction, you can see that 2 moles of NaOH react with 1 mol of H2SO4, so doing a rule of three:
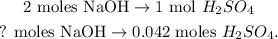
And the calculation would be:
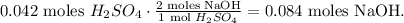
Now we can calculate the molarity using the formula:
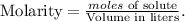
But before doing that, we need to convert 85 mL to liters. Remember that 1 liter equals 1000 mL:
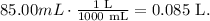
And finally, we can calculate the molarity like this:
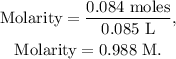
The answer is that the molarity of the base before neutralizing it is 0.988 M.