Description of the experiment
1. You must have a piece of chalk and record the weight before writing the name.
2. You write the name Valeria on a blackboard.
3. You record the weight of the chalk last, after writing the name.
Calculations:
The variables will be:
Initial mass=Mo
Final mass = Mf
Mass of chalk used=M
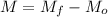
We must calculate the moles of chalk used. Since chalk is CaCO3 and its molar weight is 100.1g/mol, the moles of CaCO3 will be:
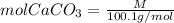
The number of atoms must be found with Avogadro's number, we will have to:
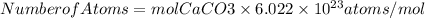
That is the description of how you should do the experiment and the calculations to find the number of atoms