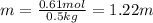To solve this question, we have to convert the grams of glucose to moles using its molecular weigth and convert the grams of water to kilograms of water using a conversion factor:
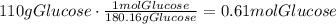
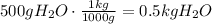
Now, divide the number of moles of glucose by the number of kg of water to find the molality of the solution: