Givens.
• The vertical initial velocity is 20 m/s.
,
• The horizontal initial velocity is 30 ms/s.
,
• The gravity is -9.8 m/s^2.
Use the following formula to find the horizontal reach of the projectile.
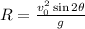
First, we need to find the initial speed using the components we already have.
![\begin{gathered} v_0=\sqrt[]{v^2_x+v^2_y}=\sqrt[]{30^2+20^2}=\sqrt[]{900+400}=\sqrt[]{1300} \\ v_0\approx36((m)/(s)) \end{gathered}](https://img.qammunity.org/2023/formulas/physics/college/tr4fqraz3bu3hdqbbh7e2woxz7e8eamus3.png)
Now we need to find the direction of the initial velocity.
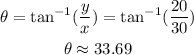
Once we have the initial velocity and its direction, use the formula to find the horizontal reach.
![\begin{gathered} R=\frac{(\sqrt[]{1300})^2\cdot\sin (2\cdot33.7)}{-9.8} \\ R=(1300\cdot(-0.99))/(-9.8) \\ R=(-1286.50)/(-9.8) \\ R=131.28m \end{gathered}](https://img.qammunity.org/2023/formulas/physics/college/kmzdvdj2b8j7ioce7bli84smidg1a1m9gg.png)
Therefore, the horizontal reach is 131.28 meters, approximately.