The wheel is circular, thus it has 360º. We can see in the picture that the rods of the wheel divide it in 6 equal part. Then each section will have:
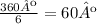
A measure of 60º on each section, then if the wheel is rotated 60º, the image will be indistinguishable from the original.
The answer is 60º