Given
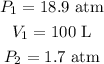
The ideal gas equation is given as,

Here, n is the number of moles, R is the universal gas constant and T is the temperature (constant).
Therefore,
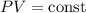
Hence,
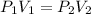
Therefore, the final volume V_2 is given as,
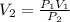
Substituting all known values,
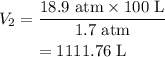
Therefore, the final volume is 1111.76 L.