Answer
1.25 mol/L
Step-by-step explanation
Given:
Volume of the solution, V = 60.0 mL
Moles of NH4Cl = 0.075 mol.
What to find:
The concentration of the solution.
Step-by-step solution.
Step 1: Convert the volume of the solution from mL to L.
You can convert the volume from mL to L by dividing the mL by 1000.
V = 60.0 mL = (60.0/1000) = 0.06 L
Step 2: Determine the concentration of the solution.
The concentration of the solution can be determined using the concentration formula below:
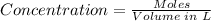
Put moles = 0.075 mol and Volume = 0.06 L into the formula:
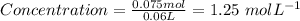
Therefore, the concentration of a 60,0 mL solution that contains 0.075 mol of NH4Cl = 1.25 mol/L