Anwer:
The equilibrium concentration of H2 is 0.0951M.
Step-by-step explanation:
1st) It is necessary to calculate the initial concentration of H2 and I2 in the Molarity unit, that is, in 1 liter of solution:
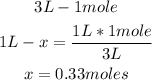
Now we know that the initial concentration of H2 and I2 is 0.33M.
2nd) Now we have to analyze the Initial concentrations of each compound, the change of each one in the reaction and the final concentration when reaching equilibrium:
3rd) Now we can replace the equilibrium expressions in the Kc equation for the given reaction:
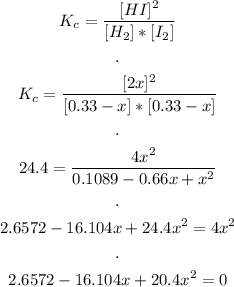
Finally, we obtain a quadratic expression that we must solve.
4th) After solving the quadratic equation, we obtain two values for x: 0.2349 and 0.5545.
In order to continue, we must choose the value of x that is less than the initial concentrations, in this case, the correct value of x is 0.2349.
Finally, we substitute the value of x (0.2349) in the equilibrium concentration, and we obtain that the equilibrium concentration of H2 is 0.0951M:
0.33 - 0.2349 = 0.0951