Based on the chemical equation the acid to base mole ratio is 1:3
First we must determine the number of moles in 100 mL 0f 0.1 M NaOH:
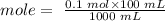
mole = 0.01 NaOH
By using the mole ratio 1:3 to determine how many moles of H3PO4 reacted
mole =0.01/3
mole = 0.0033 H3PO4
Volume of H3PO4 needed to react with NaOH is:
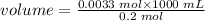
volume = 16.6 mL