1) Formula mass. We have to look for every atomic mass of the elements in the compound and multiply by its subscript.
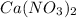
Calcium (Ca): 40.078 u
Nitrogen (N): 14.007 u
Oxygen (O): 15.999 u
Formula mass = 40.078 u + (2*14.007 u) + (6*15.999 u) = 164.08 amu.
The answer is 164.10 amu.