Given
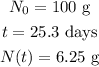
According to law of radioactive decay,
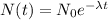
Rearranging equation in order to get rate constant,
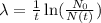
Substituting all known values,
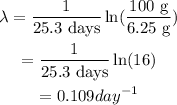
The relation between the rate constant and the half life is given as,
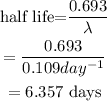
Therefore, half life of the Gold -198 is about 6.375 days.