Answer
17.1 atm
Step-by-step explanation
Given:
The initial volume, V₁ = 123 L
The initial pressure, P₁ = 16 atm
The final volume, V₂ = 115 L
What to find:
The final pressure, P₂.
Step-by-step solution:
The exercise is a volume-pressure relationship and this can be approached using Boyle's law formula.
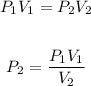
Substitute the values of the given parameters into the formula, we have
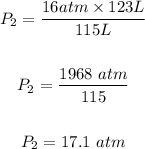
The final PRESSURE is 17.1 atm