Step 1 - Finding the stoichiometry of the reaction
The given reaction is:
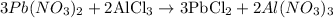
The stoichiometry of the reaction can be found by the bigger numbers that come before the formula of the substances:
3 moles of Pb(NO3)2 react with 2 moles of AlCl3 thus producing 3 moles of PbCl2 and 2 moles of Al(NO3)3
Step 2 - Using the stoichiometry to solve the question
The stoichiometry of a reaction works like a cake recipe. It defines a fixed proportion between the reactants and the products. We can thus use it to predict how much moles of Pb(NO3)2 would be needed.
In step 1, we have stated that:
3 moles of Pb(NO3)2 produce 2 moles of Al(NO3)3
Now let's set the following proportion:
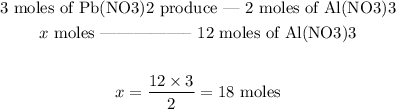
Therefore, 18 moles of Pb(NO3)2 would be required.