Answer:
The answer is "Greater than zero, and greater than the rate of the reverse reaction".
Step-by-step explanation:
It applies a rate of reaction to the balance, a forward response dominates until it reaches a constant. This process is balanced before 52 mmol of the reactant
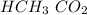 , to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.
, to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.