Answer:
Step-by-step explanation:
From this question, we are to fill in the blanks with the appropriate correct answers.
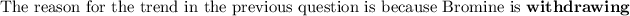
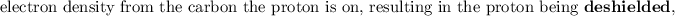
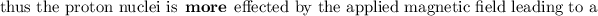
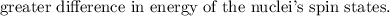
NOTE: that the electron-withdrawing groups decrease the electron density around the proton nucleus and therefore the nucleus feels the external magnetic field more. Hence, it requires a larger frequency to attain resonance(downfield).