Answer:
20.8L = Final volume of the gas
Step-by-step explanation:
Based on Charles's law, the volume of a gas is directly proportional to the temperature of the gas under pressure constant. The equation is:
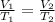
where V is volume and T absolute temperature of 1, initial state and 2, final state.
If initial volume is 22.5L, initial T = 365K and final temperature 338K:
22.5L / 365K = V₂ / 338K
20.8L = Final volume of the gas