We know that there are 20g of gold and we must calculate how many atoms there are in the sample.
First, we must use the molecular mass of the gold,
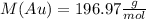
Then, we must use the next formula
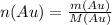
Where,
- n represents the number of molecules
- m is the given mass (20 g)
- M is the molecular mass
Now, replacing the values in the formula
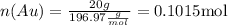
Finally, we must use that there are 6.022 * 10^23 molecules per mole
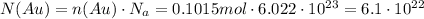
ANSWER:
6.1 * 10^22 atoms are in 20g sample of gold.