The given chemical equation is
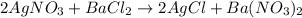
According to the equation, the stoichiometry ratio between AgNO3 and AgCl is 1:1, that is, 1 mole of AgNO3 produces 1 mole of AgCl.
So, use the molar mass of AgNO3 to find the number of moles used in the reaction. (The molar mass of silver nitrate is 169.87 g/mol.
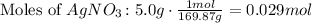
There are 0.029 moles of AgNO3. Given that the ratio is 1:1, there are produced 0.029 moles of Silver Chloride.